The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of six electrons present in the valence shell of sulfur (3s²3p⁴). Thus, sulfur has six valence electrons.
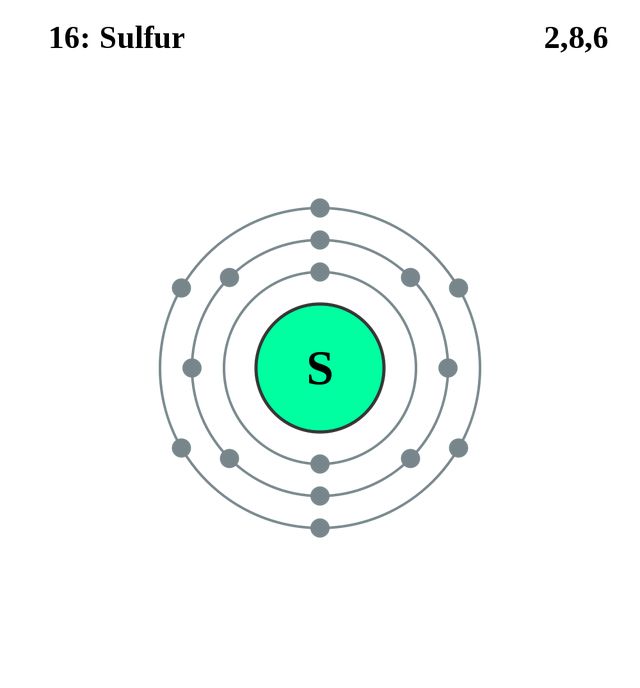
Sulfur can follow the octet rule as in the molecule SF 2. Each atom is surrounded by eight electrons. It is possible to excite the sulfur atom sufficiently to push valence atoms into the d orbital to allow molecules such as SF 4 and SF 6. The sulfur atom in SF 4 has 10 valence electrons and 12 valence electrons in SF 6. Explanation: Sulfur has six valence electrons. Valence electrons are the outermost electrons which, therefore, are located on the highest energy levels.
Click to see full answer
Beside this, what is the Lewis dot structure for sulfur?
Now let us try Lewis dot structure of Sulfide ion ( S2-). Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). Lewis dot structure will have 4 paired dots around Sulfur atom.
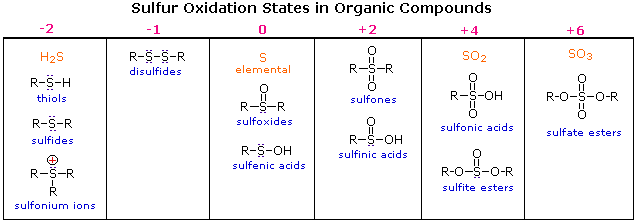
Sulfur Valence Electrons Count
Additionally, why does sulfur not follow the octet rule? An atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. This can only occur when the valence shell has enough orbitals to accommodate the extra electrons.

Beside this, what is the structure of sulfur?
Sulfur (in British English, sulphur) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent, and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature.
Is so2 bent or linear?
Valence Electrons Quizlet
Carbon dioxide is linear, while sulphur dioxide is bent (V-shaped). In sulphur dioxide, as well as the two double bonds, there is also a lone pair on the sulphur. To minimise repulsions, the double bonds and the lone pair get as far apart as possible, and so the molecule is bent.

